Ph meters
In the dynamic world of laboratory analysis, precision is key, especially when it comes to pH measurement. Our range of pH meters offers cutting-edge technology to ensure accurate and reliable results in any laboratory setting. From compact handheld devices for on-the-go testing to benchtop models with advanced features, we have the perfect pH meter to meet your specific needs.
Whether you’re working in food and beverage, pharmaceuticals, environmental testing, or research, our pH meters are designed to deliver consistent performance and user-friendly operation. With features such as automatic temperature compensation, calibration options, and data logging capabilities, you can trust our pH meters to streamline your testing procedures and enhance your overall productivity. Stay ahead in the field of laboratory analysis with our high-quality pH meters – the ultimate tools for precise pH measurement.
Types of Ph meters Your Laboratory may need
Portable
Kalstein’s portable pH meters are essential tools for on-the-go laboratory testing. Our handheld devices allow you to quickly and accurately measure pH levels in various settings, providing convenience and precision in your research or fieldwork. With user-friendly interfaces and compact designs, our portable pH meters are perfect for professionals who require flexibility and portability in their analytical tasks.
Our portable pH meters under the Medidores de pH category are equipped with advanced features such as automatic temperature compensation and electrode diagnostics, ensuring reliable and consistent results. Whether you are conducting environmental monitoring, food testing, or water quality analysis, Kalstein’s portable pH meters offer the performance and convenience you need to streamline your processes. Invest in our high-quality portable pH meters to enhance your analytical capabilities and achieve reliable measurement results on the go.
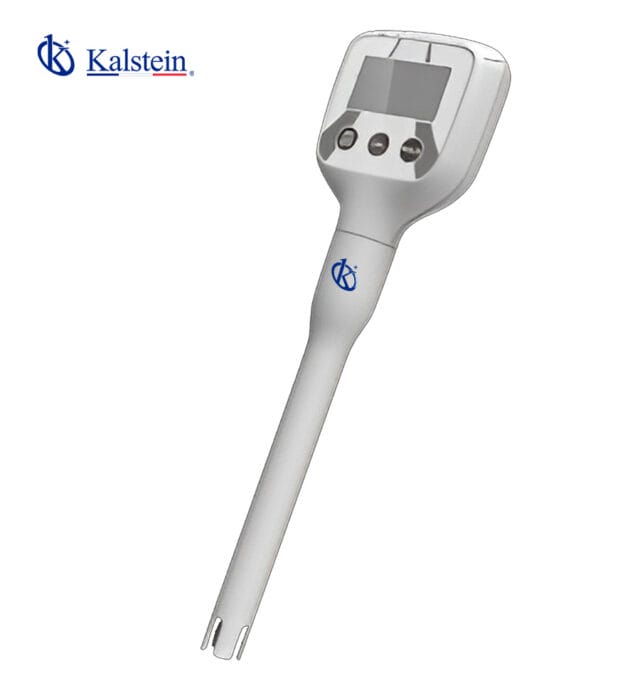
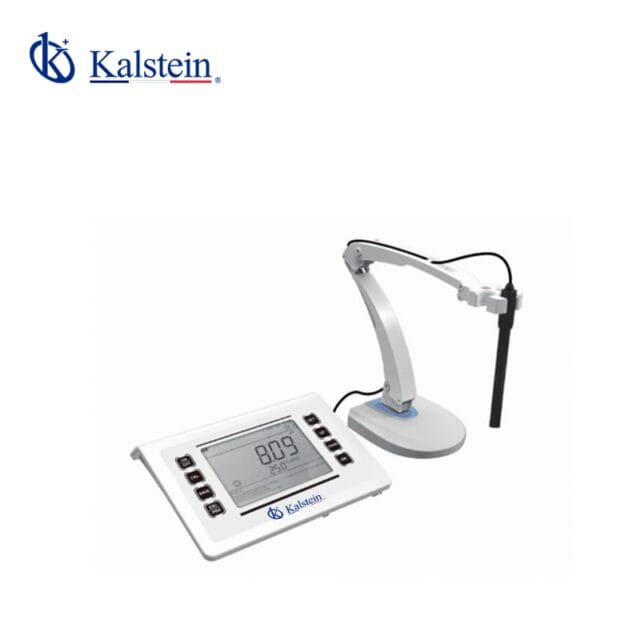
table pH meters
Sobremesa, within the realm of laboratory equipment, refers to pH meters specifically designed for desktop or tabletop use. These devices offer convenient and efficient pH measurement capabilities for various scientific and industrial applications. What sets Sobremesa pH meters apart is their compact design, user-friendly interface, and high level of accuracy in delivering precise pH readings. Users can rely on Sobremesa pH meters to monitor pH levels with ease, ensuring optimal performance and reliable results in their experiments or analyses.
Investing in a Sobremesa pH meter is a wise choice for laboratories looking to streamline their pH measurement processes without compromising on quality. With advanced features and reliable performance, Sobremesa pH meters offer a seamless experience for users seeking efficient and accurate pH measurements in a desktop format. Browse our wide selection of Sobremesa pH meters to find the perfect solution for your laboratory’s pH monitoring needs.

PH METER KALSTEIN
At Kalstein you can find the ideal Ph meters for Your Laboratory

Pocket pH Meter – ° C / ° F YR01800 – YR01800-1
Series-Pen Type- pH Meter YR01800 is Compact mini design, making it highly portable and convenient for on-the-go use. It incorporates intelligent functio...

Benchtop pH / Ion Meter YR01826
This sophisticated measurement device boasts a 5-inch LCD screen with a 150° viewing angle, ensuring clear readability from up to 5 meters away. It features a built-in microprocessor c...

YR01825 Benchtop pH / Ion Meter
This advanced measurement device features a 7-inch colored capacitive touch screen with a high resolution of 1024×600 and excellent sensitivity, providing a superior user experie...

YR01823 Benchtop pH Meter
This sophisticated measurement device is equipped with a 7-inch colored capacitive touch screen, offering high resolution and high sensitivity for an enhanced user experience. It features a built-...
Our Ph meter best seller
This sophisticated measurement device boasts a 6.5-inch LED screen with an intuitive and easy-to-use interface. It features a built-in microprocessor chip that provides smart functions such as automatic calibration, Automatic Temperature Compensation (ATC), data storage, clock display, USB output, function settings, wireless printing, and self-diagnosis information.
The instrument supports wireless Bluetooth printing, with an optional feature for real-time data transfer to mobile phones via Bluetooth. It includes a smart electrode status display to ensure precise measurements and is equipped with durable Omron light-touch keys, designed to last for over 100,000 uses.
It can automatically recognize 25 different buffers with three options: Europe & USA, NIST, and China, supporting 1, 2, and 3-point calibration. The device can store up to 1,000 sets of test data, which can be saved and transferred to a USB memory stick and easily accessed with Excel.
| Model | YR01822 |
| Measuring range | (-2.00~20.00)pH |
| Resolution | ±0.02pH |
| Accuracy | ±0.002pH |
| Auto calibration | 1, 2, 3 point |
| Buffer |
Europe & USA, NIST, China standards
|
| Input current | ≤2×10¹² A |
| Input impedance | 21×100 |
| Stability | ±0.01 pH/3h |
| Temp. compensation |
(0~100) °C, auto or manual
|
| Measuring range |
-1999.9mV~0~1999.9mV
|
| Resolution | 0.1mV |
| Accuracy | ±0.03% FS |
| Measuring range | (0~100) °C |
| Resolution | 0.1°C |
| Accuracy | ±0.5°C |
| Display |
6.5-inch LCD screen
|
| Data storage | 1000 sets |
| Power | DC 12V/1A |
| Output | USB |
| Ambient Temp. | 5~40 °C |
| Ambient Humidity | <85% |
| IP grade | IP54 |

Analysis of the best Ph meters for Your Laboratory

PHmeter: Technology and accuracy
The measurement of pH (hydrogen potential) in the laboratory is one of the most important parameters that are performed for quality control and, according to specialis...

PH meter or pH meter: How should it be used?
A pH meter is an instrument used to measure the acidity or alkalinity of a solution. The information provided by this equipment expresses the degree of acidity of an acid ...

How Do You Calibrate A Laboratory pH Meter?
pH meters are an essential tool in the laboratory, used to measure the level of acidity and basicity of a solution, which is important for performing a variety ...

What are the Benefits of Using PH Meters in Laboratories?
The PH meter is the most commonly used measuring device in laboratories; it is designed to measure the acidity or alkalinity of a solution, therefore, it offers importa...
KALSTEIN UPDATED
Guidelines for you to become an expert in Ph meters
The Ph meters equipment are essential products in Your Laboratory, we provide you with guidance and recommendations for a better use, so you can work like an expert.
PH meter: what is it used for?
What is pH and how is it measured?
PH meter vs. dissolved oxygen meter
What are the differences between: PH Meter, Ion Meter and Conductivity Meter?

Frequently asked questions from our customers about Ph meters
The delivery time of your Kalstein product will depend on the following:
- Whether the equipment you are interested in is in stock or if it needs to be manufactured.
- The type of freight you have chosen, which can be either air or sea.
- Equipment in stock:
– Delivery Time (Air): 15-30 days.
– Delivery Time (Sea): 45-60 days.
- Equipment not in stock:
– Delivery Time (Air): 30-60 days.
– Delivery Time (Sea): 60-90 days.
You can make your purchase through:
- By email: [email protected]
- By phone: +33 (0) 1 70 39 26 50
- Online shopping: Through the official Kalstein website in your country.
At Kalstein, we provide our customers with inductions and technical support through new online methods. You can visit our induction videos, technical assistance, and guidance provided by a Kalstein team through our Youtube channel (Kalstein English). HERE
Send us a direct message and one of our agents will contact you
Ph meters
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Sed dignissim placerat mauris cursus laoreet. Nam feugiat lacus ex, at fermentum sapien accumsan nec. Curabitur auctor porttitor mi non malesuada. Aenean condimentum, purus vitae rhoncus imperdiet, justo eros aliquam ipsum, at egestas leo diam eget libero.

Catalog of models of Ph meters on offer.
-

Hematology Analyzer For Veterinary YR05122-V
-
ECG Monitor 12 Channel Portable ECG Equipment YR05162
-

Electric Heating Drying Oven YR06446
-
Mini Centrifuge With Large Capacity YR012G
-

Intelligent Electric Wheelchair YR06432
-

Manual Wheelchair With Spray Function YR06431
-

Laminar Flow Cabinet YR06430 // YR06430-1
This product has multiple variants. The options may be chosen on the product page -

Counter Pressure Steam Autoclave YR06424// YR06429
This product has multiple variants. The options may be chosen on the product page -

Pulsating Vacuum Autoclave With Printer YR06418 // YR06423
This product has multiple variants. The options may be chosen on the product page -

POCT Consumables Strips YRA404
-

Substrate YRA402
-

System Washing Solution YRA401
-

Reaction Cuvette YRA400
-

Diabetes Test YRA399
-

Allergy Test YRA398
-

Anemia Test YRA395 // YRA397
This product has multiple variants. The options may be chosen on the product page
Descubre más de nuestro catálogo
Tipos de Ph meters

[Producto] A
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.

[Producto] B
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Find out more about Ph meters with our guides.
Exploring the Technical Excellence of Horizontal Cylindrical Pressure Steam Autoclaves
Product Description The Horizontal Cylindrical Pressure Steam Autoclaves, offered by Kalstein, are at the forefront of sterilization technology, designed to...
Breakthrough Class N Benchtop Autoclave Features
Product Description The Class N Benchtop Autoclave models YR03395, YR03396, YR03397, YR03398, YR03399, and YR03400 represent the cutting-edge in sterilization...
Advanced Table-Top Autoclaves: Unveiling Key Features
Product Description The Class N Table-Top Autoclave is an epitome of efficiency and convenience in the realm of sterilization technology....





